Modern Dentistry, Timeless Smiles.
From UK Lab to Global Smile: The Science Behind the Coming Enamel Regeneration Era
Language :
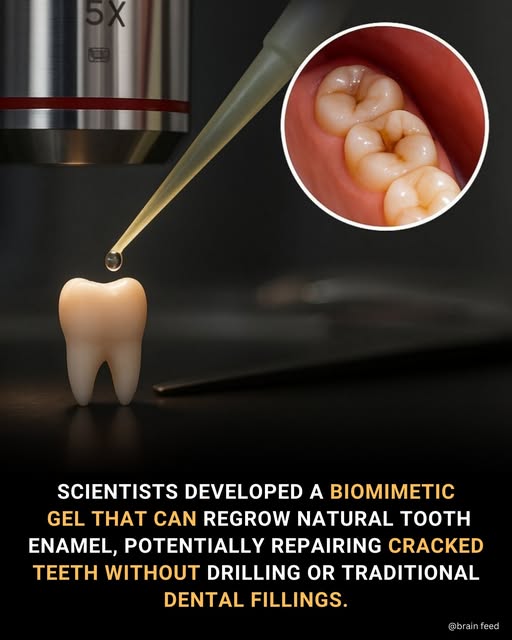
The End of Fillings? UK's Enamel Regeneration Discovery Set to Reshape Global Dentistry
A University of Nottingham Breakthrough Could Redefine Dental Care Within a Decade
In a quiet lab at the University of Nottingham, a discovery is unfolding that challenges a fundamental truth in dentistry: that tooth enamel, once lost, is gone forever. Researchers have engineered a biomimetic therapeutic gel capable of triggering the regeneration of natural tooth enamel—a feat long considered the "holy grail" of dental medicine. What began as a British innovation is now scaling rapidly, with research collaborations and parallel developments accelerating in South Korea, Japan, and the United States, signaling a pending global shift in how we treat our teeth.
The Science: Mimicking Nature's Blueprint
The core of the breakthrough lies in a protein called amelogenin. This is the architect protein that guides the formation of enamel during childhood development, after which our bodies stop producing it. The Nottingham team, led by experts in regenerative dentistry, has created a synthetic, engineered version of this protein.
How the "Regrowth Gel" Works:
-
Application: The clear gel is brushed or applied directly onto areas of demineralized or early-stage decayed enamel.
-
Nucleation: The engineered amelogenin molecules self-assemble, mimicking the natural protein matrix that exists during tooth development.
-
Mineralization: This matrix powerfully attracts free calcium and phosphate ions from saliva (and potentially from supplemented formulas), acting as a precise scaffold.
-
Crystal Growth: New hydroxyapatite crystals—the very mineral that makes up enamel—grow in the highly organized, intricate structure that gives natural enamel its legendary strength.
-
Integration: The new mineral layer bonds seamlessly with the existing tooth substrate, restoring structure and function from the inside out.
Early Data & Efficacy:
-
Regeneration Rate: Lab studies show the gel can rebuild up to 10 micrometres (µm) of new enamel in one week. While this may seem thin, early enamel lesions (pre-cavities) and areas of wear are often in the 20-50 µm range, meaning a short course of treatment could fully restore them.
-
Structural Integrity: Micro-hardness tests confirm the regenerated layer matches the mechanical properties of natural enamel, unlike previous synthetic coatings that were weaker or poorly bonded.
-
Functionality: The treatment effectively smooths rough surfaces, seals exposed dentinal tubules (reducing sensitivity), and restores a protective barrier against acid and decay.
Global Scaling: A Race for Implementation
The Nottingham discovery has ignited a collaborative international race to refine and implement the technology.
| Country | Key Institutions Involved | Focus of Research |
|---|---|---|
| United Kingdom | University of Nottingham (Pioneer), King's College London | Core gel formulation, protein engineering, initial in-vitro and in-situ trials. |
| South Korea | Seoul National University, KAIST | Nano-delivery systems, enhancing gel adhesion, combining with desensitizing agents. |
| Japan | Tokyo Medical and Dental University | Long-term durability testing, integration with minimally invasive dentistry techniques. |
| United States | University of California San Francisco, Forsyth Institute | Preparation for FDA regulatory pathways, large-scale biomaterial safety studies. |
This global effort is crucial for addressing different challenges: stability of the protein, speed of regeneration, ease of clinical application, and regulatory approval.
Implementation Roadmap & Estimated Timeline
Transitioning from lab marvel to standard dental practice is a multi-stage process. Here is a conservative, evidence-based estimate:
-
Phase 1: Optimisation & Pre-Clinical (2025-2027)
-
Goal: Enhance regeneration rate (target: 20-30 µm/week), finalize gel vehicle (varnish, strip, or tray), complete comprehensive biocompatibility and toxicity studies.
-
Status: Currently in late stages.
-
-
Phase 2: Human Clinical Trials (2028-2031)
-
Stage I (Small-Scale Safety): ~50 patients. Confirm safety in humans, ideal dosage.
-
Stage II (Efficacy): ~200-300 patients. Measure enamel rebuild on early caries and hypersensitivity.
-
Stage III (Large-Scale): ~1000+ patients across multiple centers. Compare directly to fluoride varnish and sealants.
-
-
Phase 3: Regulatory Approval & Commercialization (2032-2034)
-
UK (MHRA) & Europe (EMA): Likely first approvals, potentially as a medical device or advanced therapy.
-
USA (FDA): A longer pathway (3-5 years post-EU approval), requiring extensive data.
-
Asia (MFDS, PMDA): Could align with or follow EU timing.
-
-
Phase 4: Clinical Adoption & Impact (2035+)
-
Initial Use: Early intervention tool. Used by dentists to remineralize early "white spot" lesions, treat wedge-shaped cervical erosions, and manage severe sensitivity—replacing many uses of fluoride varnish and dentin sealers.
-
Future Potential: With advanced formulations, it could reduce or eliminate the need for drilling and composite fillings for small to moderate cavities, representing a shift to truly regenerative, preventive-restorative dentistry.
-
Data Analysis: The Potential Market & Practice Shift
-
Market Disruption: The global dental caries treatment market is worth over $30 billion. A significant portion (addressing early decay and sensitivity) could be disrupted by this technology.
-
Patient Impact: It promises a painless, drill-free alternative for millions, potentially improving oral health compliance and reducing dental anxiety.
-
Environmental Impact: Could reduce the need for composite resins (plastics) and amalgam, aligning with green dentistry initiatives.
-
Clinical Shift: Dentists would move from a "subtractive" model (drill, remove, fill) to an "additive" model (assess, apply, regenerate). Continuing education will be vital.